`=>` The Periodic Law, as we know it today owes its development to the Russian chemist, Dmitri Mendeleev (1834-1907) and the German chemist, Lothar Meyer (`1830-1895`).
`=>` Working independently, both the chemists in `1869` proposed that on arranging elements in the increasing order of their atomic weights, similarities appear in physical and chemical properties at regular intervals.
`=>` Lothar Meyer plotted the physical properties such as atomic volume, melting point and boiling point against atomic weight and obtained a periodically repeated pattern.
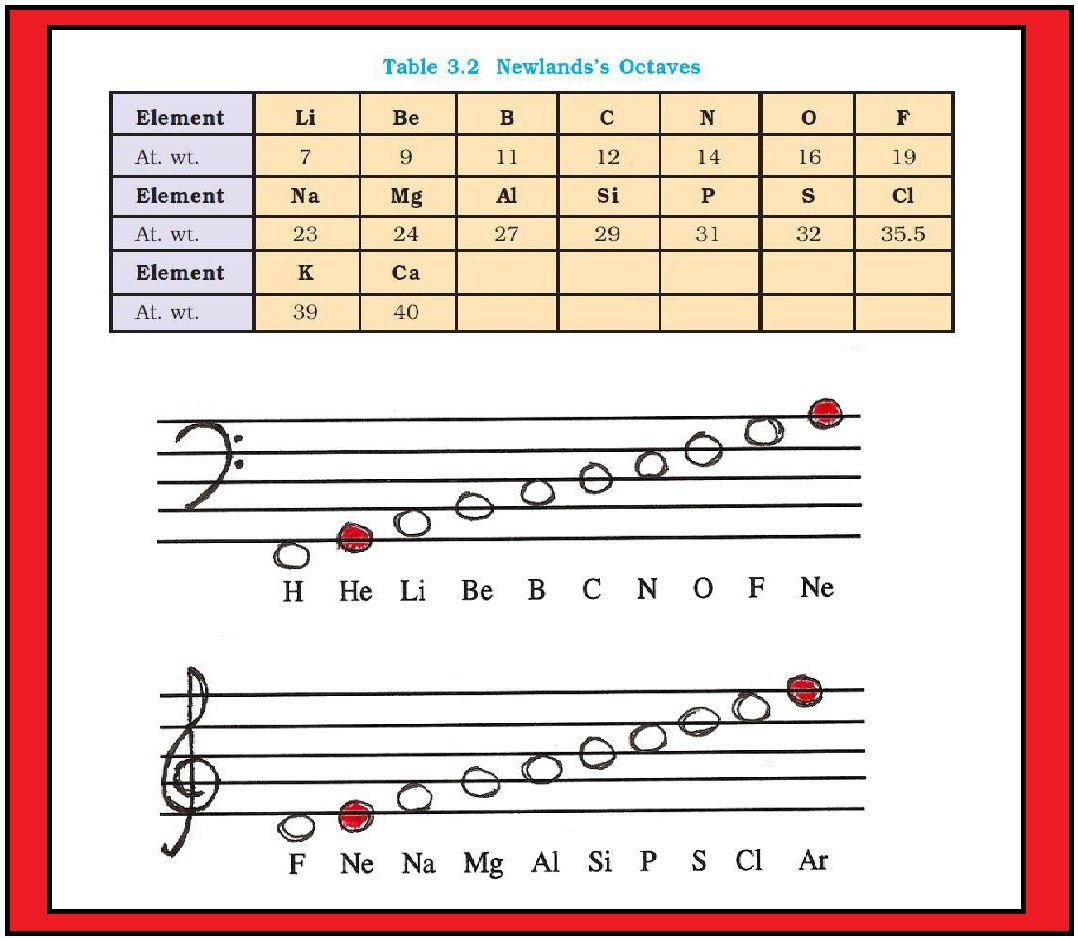
`=>` Unlike Newlands, Lothar Meyer observed a change in length of that repeating pattern.
`=>` By 1868, Lothar Meyer had developed a table of the elements that closely resembles the Modern Periodic Table.
`=>` However, his work was not published until after the work of Dmitri Mendeleev, the scientist who is generally credited with the development of the Modern Periodic Table.
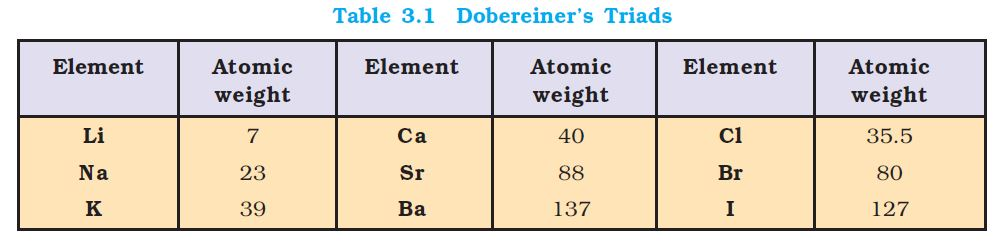
`=>` While Dobereiner initiated the study of periodic relationship, it was Mendeleev who was responsible for publishing the Periodic Law for the first time. It states as follows :
`text(The properties of the elements are a periodic function of their atomic weights)`.
`=>` Mendeleev arranged elements in horizontal rows and vertical columns of a table in order of their increasing atomic weights in such a way that the elements with similar properties occupied the same vertical column or group.
`=>` Mendeleev’s system of classifying elements was more elaborate than that of Lothar Meyer’s.
`=>` He fully recognized the significance of periodicity and used broader range of physical and chemical properties to classify the elements.
`=>` In particular, Mendeleev relied on the similarities in the empirical formulas and properties of the compounds formed by the elements.
`=>` He realized that some of the elements did not fit in with his scheme of classification if the order of atomic weight was strictly followed.
`=>` He ignored the order of atomic weights, thinking that the atomic measurements might be incorrect, and placed the elements with similar properties together.
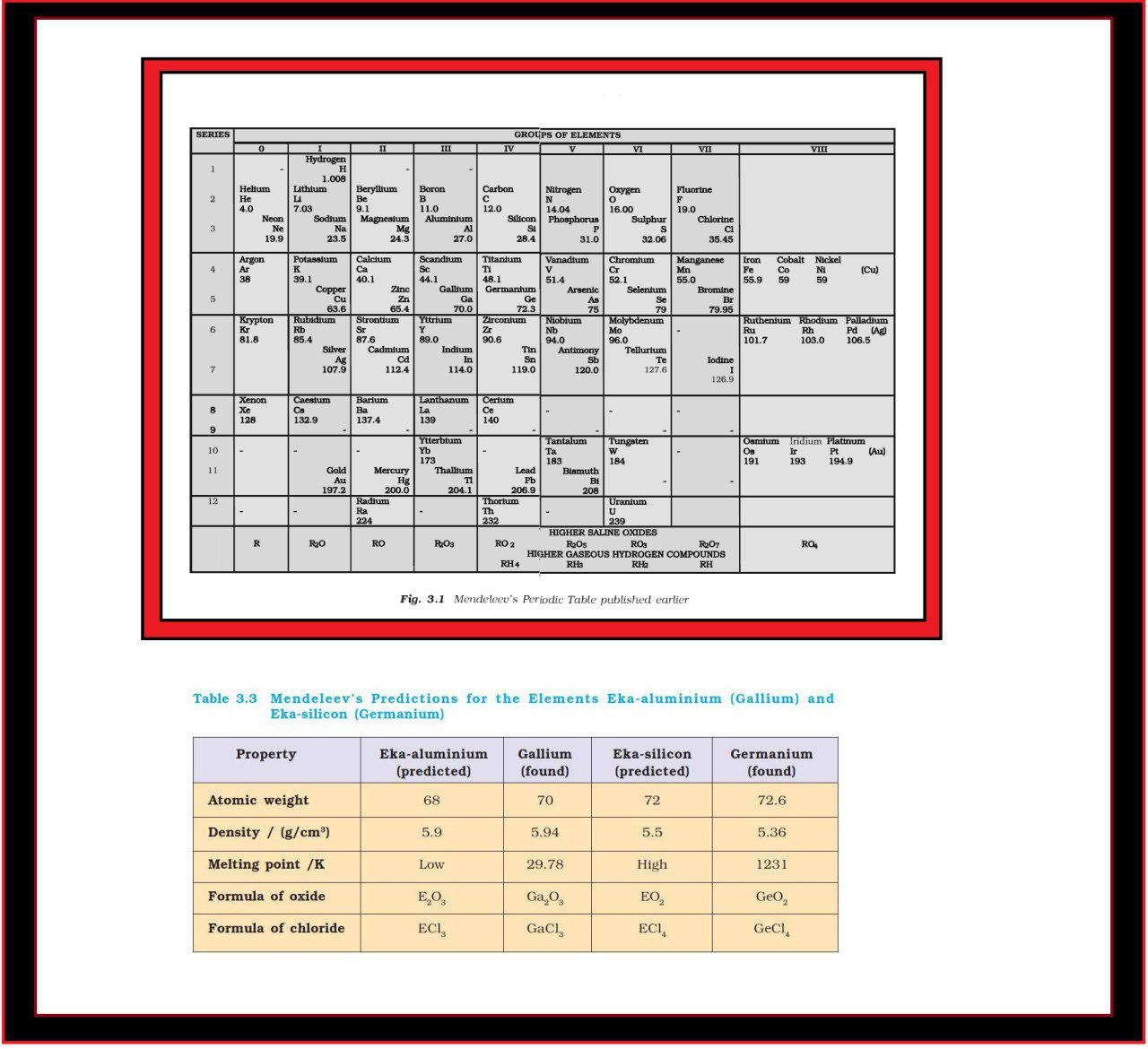
● For example, iodine with lower atomic weight than that of tellurium (Group VI) was placed in Group VII along with fluorine, chlorine, bromine because of similarities in properties (Fig. 3.1).
`=>` At the same time, keeping his primary aim of arranging the elements of similar properties in the same group, he proposed that some of the elements were still undiscovered and, therefore, left several gaps in the table.
● For example, both gallium and germanium were unknown at the time Mendeleev published his Periodic Table. He left the gap under aluminium and a gap under silicon, and called these elements Eka- Aluminium and Eka-Silicon.
● Mendeleev predicted not only the existence of gallium and germanium, but also described some of their general physical properties.
● These elements were discovered later. Some of the properties predicted by Mendeleev for these elements and those found experimentally are listed in Table 3.3.
`=>` The boldness of Mendeleev’s quantitative predictions and their eventual success made him and his Periodic Table famous. Mendeleev’s Periodic Table published in 1905 is shown in Fig. 3.1.
`=>` The Periodic Law, as we know it today owes its development to the Russian chemist, Dmitri Mendeleev (1834-1907) and the German chemist, Lothar Meyer (`1830-1895`).
`=>` Working independently, both the chemists in `1869` proposed that on arranging elements in the increasing order of their atomic weights, similarities appear in physical and chemical properties at regular intervals.
`=>` Lothar Meyer plotted the physical properties such as atomic volume, melting point and boiling point against atomic weight and obtained a periodically repeated pattern.
`=>` Unlike Newlands, Lothar Meyer observed a change in length of that repeating pattern.
`=>` By 1868, Lothar Meyer had developed a table of the elements that closely resembles the Modern Periodic Table.
`=>` However, his work was not published until after the work of Dmitri Mendeleev, the scientist who is generally credited with the development of the Modern Periodic Table.
`=>` While Dobereiner initiated the study of periodic relationship, it was Mendeleev who was responsible for publishing the Periodic Law for the first time. It states as follows :
`text(The properties of the elements are a periodic function of their atomic weights)`.
`=>` Mendeleev arranged elements in horizontal rows and vertical columns of a table in order of their increasing atomic weights in such a way that the elements with similar properties occupied the same vertical column or group.
`=>` Mendeleev’s system of classifying elements was more elaborate than that of Lothar Meyer’s.
`=>` He fully recognized the significance of periodicity and used broader range of physical and chemical properties to classify the elements.
`=>` In particular, Mendeleev relied on the similarities in the empirical formulas and properties of the compounds formed by the elements.
`=>` He realized that some of the elements did not fit in with his scheme of classification if the order of atomic weight was strictly followed.
`=>` He ignored the order of atomic weights, thinking that the atomic measurements might be incorrect, and placed the elements with similar properties together.
● For example, iodine with lower atomic weight than that of tellurium (Group VI) was placed in Group VII along with fluorine, chlorine, bromine because of similarities in properties (Fig. 3.1).
`=>` At the same time, keeping his primary aim of arranging the elements of similar properties in the same group, he proposed that some of the elements were still undiscovered and, therefore, left several gaps in the table.
● For example, both gallium and germanium were unknown at the time Mendeleev published his Periodic Table. He left the gap under aluminium and a gap under silicon, and called these elements Eka- Aluminium and Eka-Silicon.
● Mendeleev predicted not only the existence of gallium and germanium, but also described some of their general physical properties.
● These elements were discovered later. Some of the properties predicted by Mendeleev for these elements and those found experimentally are listed in Table 3.3.
`=>` The boldness of Mendeleev’s quantitative predictions and their eventual success made him and his Periodic Table famous. Mendeleev’s Periodic Table published in 1905 is shown in Fig. 3.1.